
Ksp expression for AgCl = 0.05 mol dm -3 * 0.05 mol dm -3 = 2.5 * 10 -3 mol 2 dm -6īecause calculated value for Ksp expression is greater than Ksp value of AgCl.

thereby causing the formation and precipitation of Ag 2 SO 4. Initial Cl - concentration after mixing = 0.05 mol dm -3 Initial Ag + concentration after mixing = 0.05 mol dm -3 Previously, Ag 2 SO 3 and Ag 2 SO 4 were not widely reported as corrosion products on. (b) What is the mass of the precipitate when 2.76 g of aluminum sulfate in 125 mL of solution is combined with 85.0 mL of 0. (a) Write a balanced net ionic equation for the reaction. Then we can apply Ksp expression to check whether will there be a AgCl precipitate. When solutions of aluminum sulfate and sodium hydroxide are mixed, a white gelatinous precipitate forms. Then each compound has the concentration of 0.05 mol dm -3. Let's think, when both solutions are mixed, there is no precipitate and then calculate concentration of each compound.īecause each compound is diluted by two times, concentration of NaCl and AgNO3 is reduced by half.
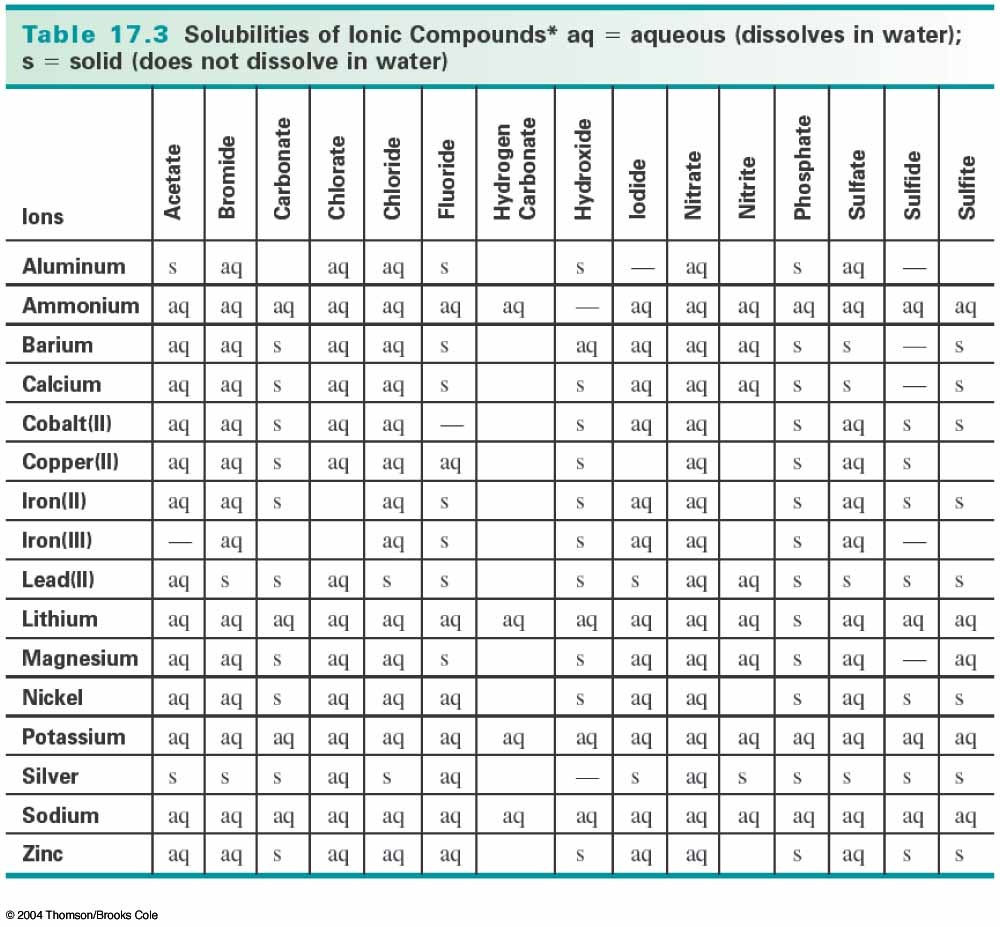
When similar volumes of NaCl and AgNO 3 are mixed, will there be a precipitate?īoth solutions have concentrations of 0.1 mol dm -3. High purity, submicron and nanopowder forms. A red or violet color of the CC1, may be caused by: BrO3-, IO3, IO,. Silver Carbonate is generally immediately available in most volumes. white AgIO3, Ag2SO3, little and possibly derived from H2S2O3, Ag2S2O3, Ag2SeO3. Carbonate compounds also give off carbon dioxide when treated with dilute acids. Required concentration to see the precipitation Silver Carbonate is a water insoluble Silver source that can easily be converted to other Silver compounds, such as the oxide by heating (calcination). If you immediately add one chemical to another, precipitate is also formed immediately.īut, remember that to be careful with silver nitrate and during the experiment nitrate because it is a very toxic chemical.
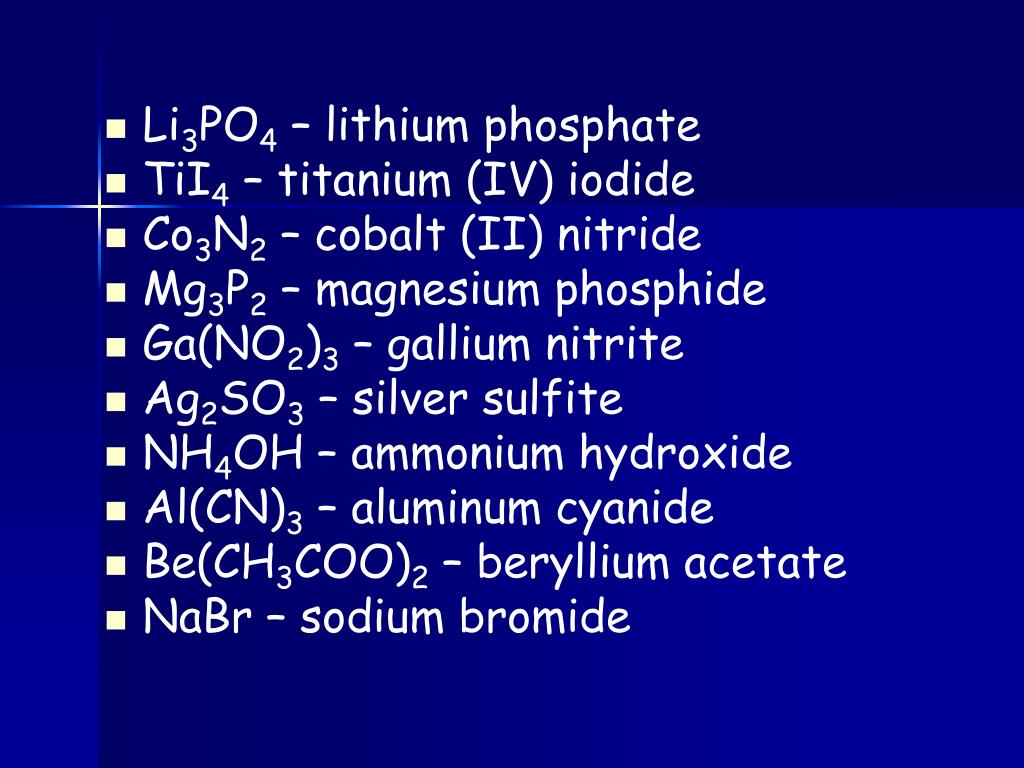
When you are slowly adding one chemical to other chemical drop by drop, at one time, you will see a white precipitate


 0 kommentar(er)
0 kommentar(er)
